Abstract

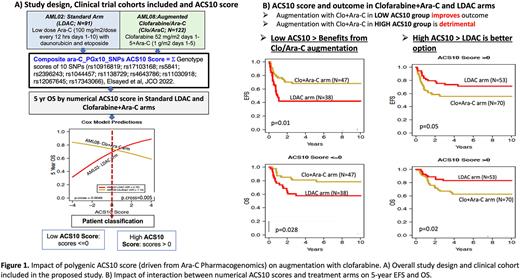
Acute myeloid leukemia (AML) is a heterogenous disease with overall suboptimal outcome. Although chemotherapy regimens that include cytarabine (ara-C) induce remission in a majority of pediatric AML patients, approximately 30% relapse and subsequently have very dismal outcome. Ara-C is a prodrug requiring activation to ara-CTP which induces leukemic cell death. Thus, inter-patient variation in each patient's ability to activate ara-C is a significant contributor to clinical outcomes. We recently performed a comprehensive pharmacogenomics evaluation of SNPs in ara-C metabolism pathway genes and developed a polygenic ara-C 10-SNP genotype (ACS10) score based on association with leukemic intracellular ara-CTP levels and clinical outcome (Elsayed et al, 2022, JCO1). Our results demonstrated that patients with low ACS10 score had poor outcomes when given standard induction including low-dose ara-C, daunorubicin and etoposide (ADE); however their outcomes were much better by augmenting therapy with either an increased dose of ara-C or the addition of gemtuzumab ozogamicin1. Conversely, patients with a high ACS10 score fared worse when given high-dose ara-C than low ara-C dose-based ADE induction (LDAC). These results suggest that pediatric AML patients may benefit from pharmacogenetic personalization of ADE therapy according to their ACS10 scores. Additionally, these results motivate research regarding the utility of ACS10 for pharmacogenetic personalization of other therapies for pediatric AML. Clofarabine is another nucleoside analog that inhibits both DNA polymerase as well as ribonucleotide reductase, thus enhancing the activity of ara-C. Recent data from the AML08 clinical trial [NCT00703820] has shown promising results with use of clofarabine with cytarabine during remission induction in pediatric AML patients.2 Therefore, we evaluated the association of ACS10 score with outcomes among 91 patients randomly assigned standard ADE induction on the AML02 clinical trial (LDAC arm) [NCT00136084] and 117 patients randomly assigned an induction of clofarabine and ara-C (Clo+Ara-C arm) on the AML08 trial [NCT00703820] (Figure 1A shows the study design). 5-year event-free survival (EFS) and overall survival (OS) of patients treated with ADE improved with increasing ACS10 score; however, for patients treated with Clo+Ara-C, improvement with increasing ACS10 score was not observed (OS shown in Figure 1A). This differential outcome association pattern suggests that thresholding the ACS10 score at 0 could be used to classify patients into low (<=0) and high (>0) ACS10 groups. Patients within low ACS10 score group had a better outcome with Clo+AraC whereas the high ACS10 score patient group fared better with ADE. In particular, there is significant improvement in EFS and OS within the low-ACS10 score patients when treated with Clo+Ara-C as compared to ADE (Clo+AraC vs. ADE; EFS HR=0.45, 95% CI: 0.23-0.82; p=0.01; OS HR=0.45, 95% CI: 0.19-0.93, p=0.028; Figure 1B).
In contrast, patients with a high ACS10 score had worse EFS and OS with Clo+AraC induction than with ADE induction (EFS HR= 1.84, 95% CI: 0.99-3.41, p=0.05; OS HR= 2.40, 95%CI: 1.13-5.13; p=0.02, Figure 1B). These results are unlikely to suffer from a strong historical comparison bias because there are not statistically significant differences in the outcomes of the randomly assigned therapies of the AML02 and AML08 trials as well as outcome by the clinical trial (EFS/OS of each arm of the two trials as well as between AML02 and AML08 trials; p=0.8, data not shown).
In summary, consistent with our recent report, these results show that for patients with Low-ACS10 genetic score prognosis is poor with standard ADE induction. However, therapy augmentation options such as Clo+AraC, ADE with high-dose Ara-C, and ADE+gemtuzumab may improve the prognosis. This calls for an action on using preemptive genotyping for selection of the most effective induction 1 chemotherapy regimen to achieve maximum clinical benefit.
References.
1. Elsayed AH, et al: Polygenic Ara-C Response Score Identifies Pediatric Patients With Acute Myeloid Leukemia in Need of Chemotherapy Augmentation. JCO 2022, PMID:34990262
2. Rubnitz JE, et al: Clofarabine Can Replace Anthracyclines and Etoposide in Remission Induction Therapy for Childhood Acute Myeloid Leukemia: The AML08 Multicenter, Randomized Phase III Trial. JCO 2019, PMID:3124652
Disclosures
Inaba:Servier: Other: Grants and Personal Fees; Amgen: Other: Grants and Personal Fees; Incyte: Other: Grants; JAZZ: Other: Personal Fees; Chugai: Other: Personal Fees. Pui:Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees; Novartis: Other: Data monitoring committee.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal